Philips Respironics Recall Information
Updated February 3, 2022
As per Philips Respironics
Beginning on 1/27/2022, any order placed with Philips Respironics for a recall replacement CPAP unit will be fulfilled with a DreamStation 2 device.
DreamStation 2 shipments come with all machine components.
Patients who had heated tubing will receive a 12mm slimline heated tube.
Patients who had standard tubing will receive a 15mm standard tube.
The following orders placed with Philips Respironics will be fulfilled with a DreamStation 1 device:
- All BiPAP orders
- All orders placed prior to 1/27/2022
Replacing your machine - What you can do:
Register your device with Phillips Respironics, if you havent already.
Philips Respironics will send a replacement DreamStation machine to the registered address. Recalled DreamStations' serial numbers end in C and the replacement machine serial numbers end in F.
Philips Respironics will give complete instructions on the replacement process and how to return the recalled device. Please read their materials carefully upon delivery.
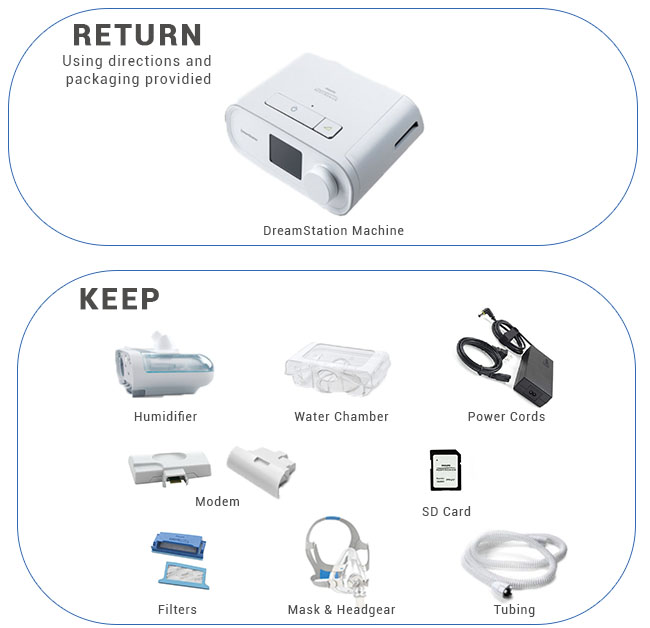
We would like to reiterate that customers should keep the following items as they will connect to the new machine:
- Humidifier
- Water Chamber
- Modem
- SD Card
- Power Cords
- Supplies: Filters, Headgear, Mask and Tubing
Philips Respironics will not be replacing the items listed above. They only require the DreamStation machine portion be returned. The customer will be responsible for replacing any additional items returned. Do not send any items until instructed by Philips Respironics.
Registration Instructions
The first step is to register your device so Philips can facilitate sending a replacement.
- To register your device, please call 1-877-907-7508 or visit www.philips.com/src-update
- Follow the link on the right side of the page that says “Begin Registration Process”
- You will need to enter your device serial number. The serial number is located on the bottom of the CPAP devices, starts with the letter J. Please remember to empty your humidifier before turning over your device to read the number.
If you have already registered your device, please call 1-800-644-3324 for replacement status and see below for important note.
Replacement Process
Philips Clincial Information - Sleep and Respiratory Care Update
Updated July 8, 2021
FDA Safety Communication
The U.S. Food and Drug Administration (FDA) has just issued a Safety Communication regarding Philips’ recent recall of certain respiratory devices.
The FDA communication summarizes major issues and considerations related to the recall, and encourages patients using BiPAP and CPAP machines to discuss treatment options and alternatives with their healthcare providers, including “Continuing to use your affected device, if your health care provider determines that the benefits outweigh the risks identified in the recall notification.”
The FDA also notes that they are working with Philips to evaluate the issue, the scope of the recall, and appropriate mitigation strategies. The Agency is also analyzing medical device reports related to the affected devices over the period of 2009-2021 for reports that could be related to this issue.
The communication also urges patients to register their devices to Philips’ website and also includes a link to the Medwatch Reporting Form where individuals can report problems with their devices directly to the FDA.
Suppliers are encouraged to share these links with their patients. By registering their devices with Philips, patients will help provide useful data on the total number of devices affected.
- The registration link can be found under the "Patients, Users, and Caregivers" heading on Philips recall website. Here is a direct link.
- FDA MedWatch Voluntary Reporting Form.
Philips Respironics Recall Notification
Updated June 23, 2021
Philips Respironics, the manufacturer of PAP or Trilogy ventilation devices has announced a voluntary recall of their equipment due to possible health risks. Not all devices are affected by this recall, please visit www.philips.com/src-update for:
- Recall notification
- List of affected devices
- Recommendations from Philips Respironics
It is our understanding that Philips Respironics, in collaboration with a third- party processor is formulating a plan to repair or replace your unit. Unfortunately, Health System Services will not be allowed to repair or replace your unit directly.
We apologize, but this is all the information we have at this time. This webpage will be updated as we receive more information from Philips. Please follow patient instructions and see FAQ below:
